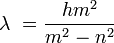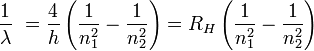<Back to Index>
- Mathematician and Physicist Johann Jakob Balmer, 1825
- Physicist Anders Jonas Ångström, 1814
PAGE SPONSOR

Johann Jakob Balmer (May 1, 1825 – March 12, 1898) was a Swiss mathematician and mathematical physicist.
Balmer was born in Lausen, Switzerland, the son of a Chief Justice also named Johann Jakob Balmer. His mother was Elizabeth Rolle Balmer, and he was the oldest son. During his schooling he excelled in mathematics, and so decided to focus on that field when he attended university.
He studied at the University of Karlsruhe and the University of Berlin, then completed his Ph.D. from the University of Basel in 1849 with a dissertation on the cycloid. Johann then spent his entire life in Basel, where he taught at a school for girls. He also lectured at the University of Basel. In 1868 he married Christine Pauline Rinck at the age of 43. The couple had a total of six children.
Despite being a mathematician, he is not remembered for any work in that field; rather, his major contribution (made at the age of sixty, in 1885) was an empirical formula for the visible spectral lines of the hydrogen atom, the study of which he took up at the suggestion of Eduard Hagenbach also of Basel. Using Ångström's measurements of the hydrogen lines, he arrived at a formula for computing the wavelength as follows:
for n = 2, h = 3.6456×10−7 m, and m = 3, 4, 5, 6, and so forth.
In his 1885 notice, he referred to h (now known as the Balmer constant) as the "fundamental number of hydrogen." Balmer then used this formula to predict the wavelength for m = 7 and Hagenbach informed him that Ångström had observed a line with wavelength 397 nm. Two of his colleagues, Hermann Wilhelm Vogel and William Huggins, were able to confirm the existence of other lines of the series for the spectrum of hydrogen of white stars.
Balmer's formula was later found to be a special case of the Rydberg formula, devised by Johannes Rydberg.
with 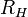 being the Rydberg constant for hydrogen,
being the Rydberg constant for hydrogen, 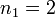 for Balmer's formula, and
for Balmer's formula, and 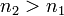 .
.
A full explanation of why these formulas worked, however, had to wait until the presentation of the Bohr model of the atom by Niels Bohr in 1913.
Johann Balmer died in Basel.

Anders Jonas Ångström (13 August 1814, Lögdö – 21 June 1874) was a Swedish physicist and one of the founders of the science of spectroscopy.
Born in Medelpad, he moved to, and was educated at Uppsala University, where in 1839 he became docent in physics. In 1842 he went to the Stockholm Observatory to gain experience in practical astronomical work, and the following year he was appointed keeper of the Uppsala Astronomical Observatory.
Becoming interested in terrestrial magnetism he made many observations of magnetic intensity and declination in various parts of Sweden, and was charged by the Stockholm Academy of Sciences with the task, not completed till shortly before his death, of working out the magnetic data obtained by the Swedish frigate "Eugénie" on her voyage round the world in 1851 - 1853.
In 1858 he succeeded Adolph Ferdinand Svanberg in the chair of physics at Uppsala. His most important work was concerned with the conduction of heat and with spectroscopy. In his optical researches, Optiska Undersökningar, presented to the Royal Swedish Academy of Sciences in 1853, he not only pointed out that the electric spark yields two superposed spectra, one from the metal of the electrode and the other from the gas in which it passes, but deduced from Leonhard Euler's theory of resonance that an incandescent gas emits luminous rays of the same refrangibility as those it can absorb. This statement, as Sir Edward Sabine remarked when awarding him the Rumford medal of the Royal Society in 1872, contains a fundamental principle of spectrum analysis, and though overlooked for a number of years it entitles him to rank as one of the founders of spectroscopy.
From 1861 onwards he paid special attention to the solar spectrum. His combination of the spectroscope with photography for the study of the solar system resulted in proving that the sun's atmosphere contains hydrogen, among other elements (1862), and in 1868 he published his great map of the normal solar spectrum in Recherches sur le spectre solaire, including detailed measurements of more than 1000 spectral lines, which long remained authoritative in questions of wave length, although his measurements were inexact by one part in 7000 or 8000, owing to the meter he used as a standard being slightly too short.
He was the first, in 1867, to examine the spectrum of the aurora borealis, and detected and measured the characteristic bright line in its yellow green region; but he was mistaken in supposing that this same line, which is often called by his name, is also to be seen in the zodiacal light.
He was elected a member of a number of learned societies, including the Royal Swedish Academy of Sciences in 1850, the Royal Society in 1870 and the Institut de France in 1873.
His son Knut (1857 — 1910) was also a physicist.
He died in Uppsala on 21 June 1874.
The ångström unit (1 Å = 10−10 m) with which the lengths on a scale of the wavelength of light or interatomic spacings in condensed matter is measured are named for him. The unit is used in crystallography as well as spectroscopy.
The crater Ångström on the Moon is named in his honor.
One of the main building complexes of Uppsala University, the Ångström Laboratory, is named in his honor.